Nanobiotics: AI for discovering where and how nanoparticles bind with proteins
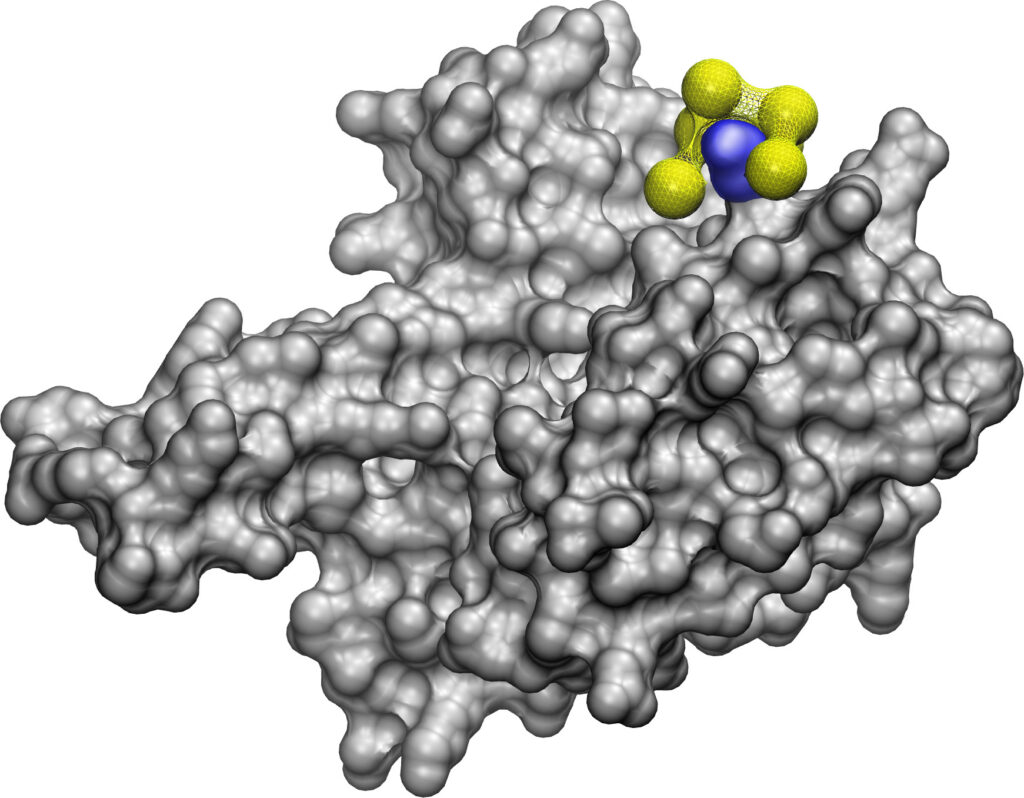
Identifying whether and how a nanoparticle and protein will bind with one another is an important step toward being able to design antibiotics and antivirals on demand, and a computer model developed at the University of Michigan can do it. The new tool could help find ways to stop antibiotic-resistant infections and new viruses—and aid in the design of nanoparticles for different purposes.
“Just in 2019, the number of people who died of antimicrobial resistance was 4.95 million. Even before COVID, which worsened the problem, studies showed that by 2050, the number of deaths by antibiotic resistance will be 10 million,” said Angela Violi, an Arthur F. Thurnau Professor of mechanical engineering, and corresponding author of the study that made the cover of Nature Computational Science.
“In my ideal scenario, 20 or 30 years from now, I would like—given any superbug—to be able to quickly produce the best nanoparticles that can treat it,” she said.
Much of the work within cells is done by proteins. Interaction sites on their surfaces can stitch molecules together, break them apart and perform other modifications—opening doorways into cells, breaking sugars down to release energy, building structures to support groups of cells and more. If we could design medicines that target crucial proteins in bacteria and viruses without harming our own cells, that would enable humans to fight new and changing diseases quickly.
The new model, named NeCLAS, uses machine learning—the AI technique that powers the virtual assistant on your smartphone and ChatGPT. But instead of learning to process language, it absorbs structural models of proteins and their known interaction sites. From this information, it learns to extrapolate how proteins and nanoparticles might interact, predict binding sites and the likelihood of binding between them—as well as predicting interactions between two proteins or two nanoparticles.
“Other models exist, but ours is the best for predicting interactions between proteins and nanoparticles,” said Paolo Elvati, an associate research scientist in mechanical engineering.
AlphaFold, for example, is a widely used tool for predicting the 3D structure of a protein based on its building blocks, called amino acids. While this capacity is crucial, this is only the beginning: discovering how these proteins assemble into larger structures and designing practical nanoscale systems are the next steps.
“That’s where NeCLAS comes in,” said Jacob Saldinger, a doctoral student in chemical engineering and first author of the study. “It goes beyond AlphaFold by showing how nanostructures will interact with one another, and it’s not limited to proteins. This enables researchers to understand the potential applications of nanoparticles and optimize their designs.”
The team tested three case studies for which they had additional data:
- Molecular tweezers, in which a molecule binds to a particular site on another molecule. This approach can stop harmful biological processes, such as the aggregation of protein plaques in diseases of the brain like Alzheimer’s.
- How graphene quantum dots break up the biofilm produced by staph bacteria. These nanoparticles are flakes of carbon, no more than a few atomic layers thick and 0.0001 millimeters to a side. Breaking up biofilms is likely a crucial tool in fighting antibiotic-resistant infections—including the superbug methicillin-resistant Staphylococcus aureus (MRSA), commonly acquired at hospitals.
- Whether graphene quantum dots would disperse in water, demonstrating the model’s ability to predict nanoparticle-nanoparticle binding even though it had been trained exclusively on protein-protein data.
While many protein-protein models set amino acids as the smallest unit that the model must consider, this doesn’t work for nanoparticles. Instead, the team set the size of that smallest feature to be roughly the size of the amino acid but then let the computer model decide where the boundaries between these minimum features were. The result is representations of proteins and nanoparticles that look a bit like collections of interconnected beads, providing more flexibility in exploring small scale interactions.
“Besides being more general, NeCLAS also uses way less training data than AlphaFold. We only have 21 nanoparticles to look at, so we have to use protein data in a clever way,” said Matt Raymond, a doctoral student in electrical and computer engineering and study co-author.
In another recent study published in ACS Applied Nanomaterials, the team went deep on staph’s biofilm. A biofilm serves as a sort of fortress, helping bacteria stay lodged on a surface and forming a protective barrier against antibiotics.
The team used molecular dynamics simulations to discover how a certain protein fragment, or peptide, that staph produces can self-assemble into a network that forms a major part of the biofilm. By comprehending how it assembles, they can better understand how to prevent it from forming or break it apart.
Detailed studies like these are also needed to discover whether a nanoparticle can achieve its prospective purpose, but they require a lot of computing time and aren’t manageable unless researchers have some idea about where binding will occur. This way, those surfaces can be set close together so that the simulation doesn’t waste weeks or months on random motions that try different surfaces against one another.
“Without experimental data, you don’t have a starting point for how these proteins and nanoparticles will interact. But now NeCLAS can tell me which parts should be closer together at the beginning of the simulation,” said Chloe Luyet, a doctoral student in chemical engineering who runs molecular dynamics simulations in the Violi group.
She is co-first-author of the study in ACS Applied Nanomaterials, along with Elvati and Yichun Wang, who had been a postdoctoral scholar on the Nanobiotics Blue Sky project that Violi led starting in 2019. Other core members of the nanobiotics team, in addition to Elvati, are co-authors of the study: J. Scott VanEpps, an assistant professor of emergency medicine, and Nicholas Kotov, the Irving Langmuir Distinguished University Professor of Chemical Sciences and Engineering and co-corresponding author with Violi for his group’s work confirming the findings with experiments.
Next, the team intends to explore other biofilms and microorganisms, including viruses.
The Nature Computational Science study was funded by Michigan Engineering’s Blue Sky Initiative, the Army Research Office and the National Science Foundation. The ACS Applied Nanomaterials study was funded by Blue Sky and the Department of Defense Advanced Research Projects Agency.
Violi is also a professor of electrical and computer engineering and biophysics. Kotov is also the Joseph B. and Florence V. Cejka Professor of Chemical Engineering and a professor of macromolecular science and engineering. VanEpps is also an assistant professor of macromolecular science and engineering
 MENU
MENU 
